Markets today by TradingView
The Definitive Who’s Who of the 2025 Papal Conclave
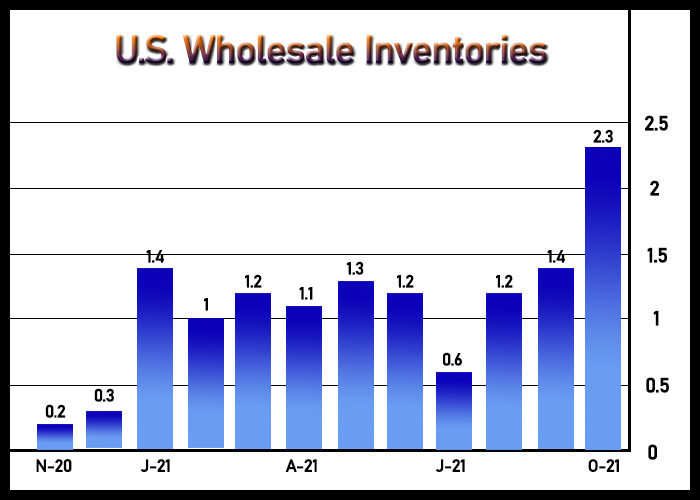
The World Is Revalidating Itself
Conclave: How A New Pope Is Chosen
Tariffs, Trump, and Other Things That Start With T – They’re Not The Problem, It’s How We Use Them
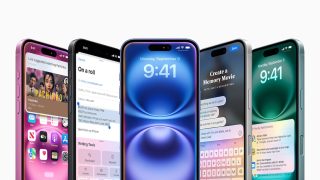
Apple debuts iPhone 16 Pro and iPhone 16 Pro Max
Apple introduces iPhone 16 and iPhone 16 Plus
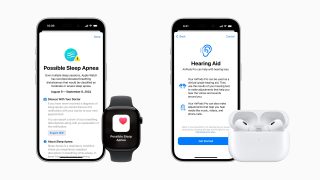
Apple introduces AirPods 4 and the world’s first all-in-one hearing health experience with AirPods Pro 2
Apple introduces groundbreaking health features to support conditions impacting billions of people
Unwind With Nature With These Luxurious Handmade Artisan Soaps Inspired By The British Countryside
Of Nuggets And Tenders. To Know Or Not To Know, Is Not The Question. How To Become, Is.
Latest News
Business
Sign Up For Our Newsletters
Economy
Market Overview
World markets by TradingView
Stock Market Today
Stock Market Today by TradingView
Trending Posts
Spotlight
Introducing Surface Laptop 5G: Seamless connectivity, built for business
We’re excited to announce the expansion of our Surface Copilot+ PC portfolio for business customers. The new Surface Laptop 5G, 13.8-inch powered by Intel Core Ultra (Series 2)…
Press Start (Or Hit Enter)! Your Go-To Loadout for Streamers and Gamers.
While streaming is defined as broadcasting a video and most of the time includes audio to a wide audience over the internet. Some consider it as a full-time job. From professional chess…
Samsung Galaxy Z Fold7: Raising the Bar for Smartphones
The most advanced Galaxy Z series yet, seamlessly blending precision engineering and powerful intelligence to elevate everyday interactions – all in its thinnest and lightest design to date…
The Summer Adventures : Camping Essentials
If the smoldering heat is bothering you, better make some plans for some quality time spending with family or friends. A camping trip on the nearest national park, official camp sites, or wild…
A Father’s Day Gift for Every Pop and Papa
It’s Father’s day and also Men’s Health Month. What better way to appreciate these than take care of our dear father (or father figure). There are many ways to show…
The Summer Adventures : Hiking and Nature Walks Essentials
Summer is almost here. Or we should say, that summer is here, but the actual weather must’ve been late to wake up. As with any change in season, its also accompanied with a change in…
The Unexpected Pi-Fect Deals This March 14
If you love pie, you might be lost and found this by mistake. But, if you love the mathematical constant number Pi (3.14) and looking for deals, then stay a while and check out these…
10 Physical Nintendo Switch Game Deals on MAR10 Day!
Happy Mar10 Day! We happen to have stumbled with discounts on Amazon this amazing day. Here are the 10 games you should get while they are on sale. Starting off with a Mario game. Super Mario…
Top Presidents’ Day Deals 2025 on Amazon
It’s Presidents’ day! Officially it’s the day to honor George Washington’s birthday but now it also celebrates all the U.S. presidents. Here are some amazing deals from…
Best Valentine’s Day Gifts That Will ‘Heart’ly Disappoint You.
Valentines is fast approaching, and while you might think the tradition of gift giving has faded, you are wrong. In the United States alone, it has grown continuously throughout the years.…
Explore
Kitchen Knives : The Surgeons of Cooking – Best All-Around Picks in 2025
One of the most essential tools in any kitchen, and the one that should always be sharpest, is the knife. From cutting, slicing, chopping, dicing, filleting, to deboning, a good knife handles…
NVIDIA Blackwell Ultra Sets the Bar in New MLPerf Inference Benchmark
At the AI Infrastructure Summit, NVIDIA’s Ian Buck introduces a reference design and partner-driven strategy to transform global infrastructure for high-performance, energy-efficient AI. At…
Apple unveils iPhone 17 Pro and iPhone 17 Pro Max, the most powerful and advanced Pro models ever
September 9, 2025PRESS RELEASE With an all-new design powered by A19 Pro, iPhone 17 Pro features the best-ever performance, camera systems, and battery life in an iPhone CUPERTINO, CALIFORNIA…
Apple debuts iPhone 17
September 9, 2025PRESS RELEASEApple debuts iPhone 17 iPhone 17 features the innovative Center Stage front camera, a bigger and brighter new display with ProMotion, and the…
Food Processor: The Swiss Army Knife of the Kitchen – Best All-Around Picks in 2025
Cooking has never been the same since we got a food processor. Honestly, it feels almost as revolutionary as when the internet first arrived. Okay, maybe that’s a bit of an exaggeration, but…
Meet Samsung Galaxy Tab S11 Series: Packing Everything You Expect From a Premium Tablet
Galaxy Tab S11 series delivers the latest Galaxy AI features and a more powerful Samsung DeX experience — thin and light tablets designed for effortless productivity Samsung Electronics today…